This article will cover the basic rules of the IUPAC nomenclature of organic compounds. IUPAC naming is the method of giving names to compounds approved by the International Union of Pure and Applied Chemistry.
Images in this article are taken from PLAY Chemistry YouTube channel.

So to start with there are 3 main rules of IUPAC nomenclature of organic compounds
- Main chain selection
- Numbering
- Naming

1- Main chain selection:
Main chain selection should be the first thing to understand in IUPAC nomenclature of organic compounds while naming hydrocarbons (organic compounds), because once you understand which one is the main chain in the compound, you can start naming it. So to select the main chain, there are the following things to keep in mind.
- -Longest chain rule
- -Difficult longest chain
- -When there is a double or Triple bond
- -When there is a functional group
- -When there is a tie (more branches)
- -Terminating chain
Longest chain rule:
For simple compounds like these, the longest chain of carbon should be selected directly as the main chain.
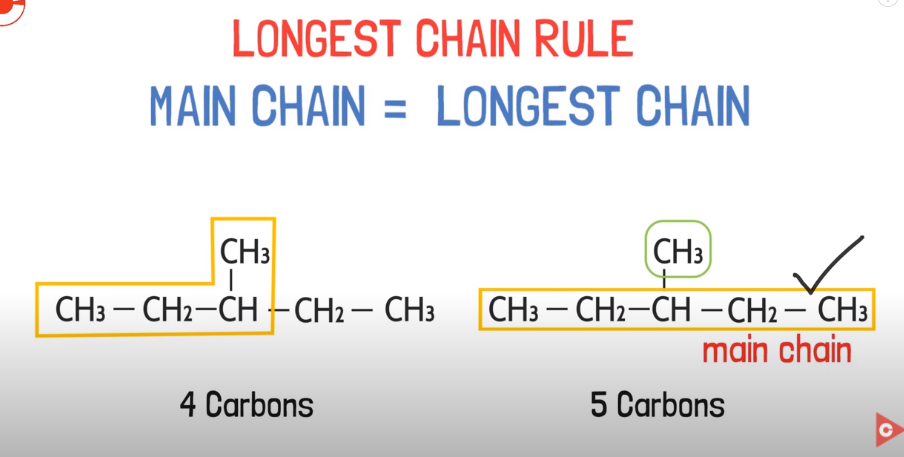
Difficult longest chain:
Keep in mind that doesn`t mean that main chain should be written in single line, there maybe different ways of creating chains like this. So longest chain of carbon will be automatically selected as main chain.

When there is a double or triple bond:
If there is double or triple bond in a compound than you have to include that bond in your main chain, even if it is not the longest chain of carbon.

When there is functional group in Compound:
If there is a functional group in the compound at the end, then like a previous rule we will not consider the longest carbon chain, instead, we will take our functional group in main chain while selecting.
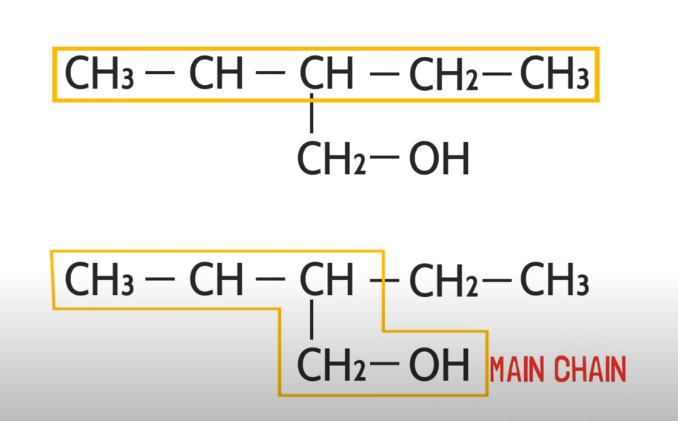
When there are branches:
If there is a tie in longest chain we will have to consider that chain having more number of side chain, like in the example below there are two possibilities one with single side chain, second one with double side chain , so we will go with the one with 2 side chains.

Terminating functional group:

When there are groups like these at the end they are called terminating functional group generally with oxygen. So like previous rules we will have to include it in our chain and make it our primary main chain of the organic compound.

2- Numbering:
Now after selecting the main chain the second thing that we need to select which one should be numbered as the first carbon, do complete numbering of your chain then we can name that compound according to that because in organic chemistry name should describe the complete compound including the position of side chains and functional groups.
So here are few rules that you should consider while numbering your compound:
- -Nearest locant (side-chain double bond functional grp)
- -Double bond > side chain
- -Double > triple & lowest locant
- -Fn > double bond
Nearest locant rule:
Locant is the location, when you have a functional group or side chain you have to consider the location of the side group or chain in order to number the carbons. You have to consider that carbon from where the location of that side locant is nearest like this:
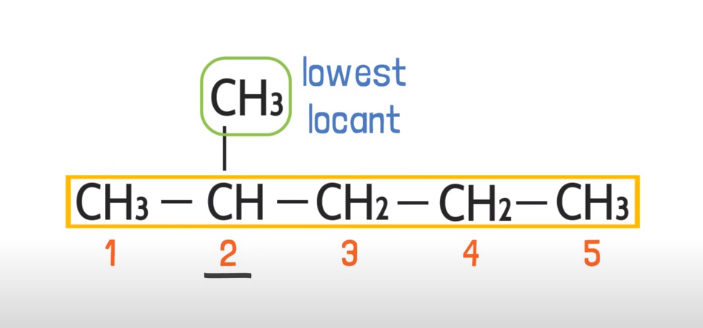
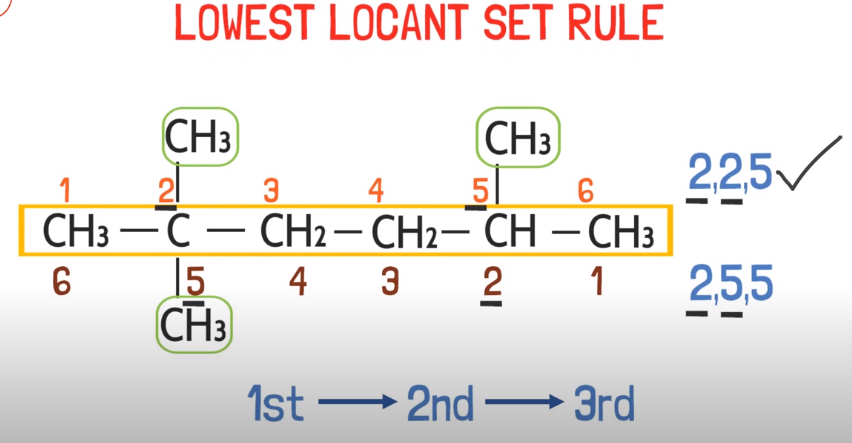
Double bond > side chain
When there is a double bond in the carbon chain , you have to consider it before the side chain or locant and make it nearest to the first carbon, so we will number our chain like this:

Double > triple
When both double and triple bonds are together than we will pick that side of chain where both bonds come closer like this:

But when there is a tie between them then we will go with double bond as more priority than triple bond while naming like this:

Functional Group > double bond
Now when there is a functional group in the bond then we will take that double bond aside and functional group will be given a priority while number and we will make it closer to the number 1

3- Naming:
It’s time to name the chain actually, so long story short simply follow these rule and your compound will have its I.U.P.A.C name
- -Side chain and Main chain
- -Repeating side chain – di tri tetra
- -Alkane Alkene Alkyne
Side chains and main chains:
The first thing is we already have our main chain, that name will come after the side chain, so we will name side chains as methyl ethyl, etc and then put our main chain in front of it, then we will put the numbers (after numbering) and add the number where that side chain is connected with main chain carbon, like this:

With that done most of the simple hydrocarbons with only hydrogen and carbon in organic chemistry will be named.
Now if here is the list of how you name your compounds:
If there is one carbon Meth will be added
For two carbons Eth
- 3- Prop
- 4- But
- 5- Pent
- 6- Hex
- 7- Pent
- 8- Oct
- 9- Non
- 10- Dec
Repeating side chains:
If there are multiple side chains with the same name (methyl), then we will not name them twice, we can add di – tri or tetra with that name just like this:

So if there are two propane in a compound we will write di propane and so on.

Alkane Alkene Alkyne:
Now with all that done IUPAC nomenclature of organic compounds, there will be the last thing in simple IUPAC naming (remember there are a lot of more things in IUPAC naming like acetones, aldehydes, etc but we are only naming simple one)
If there is a single bond only in the compound the last name will be ANE like methane
If there is a double bond (one or more than one) then the last name will be ENE
If there is a triple bond (one or more than one) then the last name will be YNE
Examples:

If there are more than one double or triple bond we have to add the number with ene or yne like 3-ene 5-yne

Also, remember double bond or E comes before triple bond Y. So in alphabetical order double bond will be numbered and named first like
Di methyl pent 3-ene 4-yne

Now if a compound have 6 carbons and one double bond its name will be Hex + ene = hexane
With all that done you can name all simpler hydrocarbons, we highly suggest you to practice these first then start with advanced IUPAC naming of chemistry. Good luck
For more keep following go techies.

